TOOLKIT. COURSE: PERMANENT MAKEUP REMOVAL SYSTEM
TOOLKIT. COURSE:
PERMANENT MAKEUP REMOVAL SYSTEM
CHEMICAL COMPOSITION OF THE REMOVER
If you study this material, then you are concerned about the process of removing permanent makeup, or perhaps you are already tired of the removal and do not know where to move on and what to do.
The actual problem is not even how, but how to remove it? Laser equipment seems to many an instant solution to the problem, but is it really so?
I will be frank: not all cases can be solved with a laser.
With salmon eyebrows (yellowish-orange) — laser equipment does not make sense to use. White-yellow, white-orange eyebrows, pinkish-orange cannot be removed with a laser. These are meaningless procedures that, in addition, the client needs to pay for.
I note a point that under the influence of laser equipment, the pigment valency changes, and if the composition contains a mineral dye, then the mineral components are removed first.
Also, when using laser equipment, we can observe a color change (pigment inversion).
Pigment residues (organic dye) are best removed chemically (remover). And here the question is, which manufacturer to choose? Masters want to know which manufacturer and composition is better.
In my work I use different companies. My choice depends on the specific case with the client. In order to understand which drug to use, it is necessary to understand the laws of coloristics, own the composition of the pigment, and also be able to apply various removal technologies in different parts of the body.
In more detail, together with Hook Natalia we train masters and provide valuable methods and protocols for effective removal.
QUALITATIVE REMOVAL = pigment + active substance
The main types of chemical removers that are used to correct makeup: salt, acid and alkaline compositions.
Acid: single-acid, two-acid, multi-acid.
Acidic acids are also divided into single-acid, two-acid, and three-acid ones.
Alkaline, as a rule, only single-phase.
Sometimes alkaline are called oxide — the main active ingredient in the composition is calcium oxide (quicklime) CaO.
Alkaline (let’s open a secret) do not work with metal oxides, since the alkali cannot interact with them.
I recommend giving preference to salt and acid formulations.
Saline
can be combined with acidic, and alcohol — can be used to soften the color of the pigment.
I give priority to acid compounds. It is important for the master:
1) understand what solution enters the skin;
2) trauma to the skin — is of particular importance (the correct removal technique).
In order to use a specific composition at the desired removal site, it is necessary to undergo training: “The course of skin restoration” (the training is conducted by Natalia Hook). When using a different composition — a different result in specific cases.
Alkaline
unlike others, they behave endlessly
If the master uses a strong alkaline composition, injuries can be terrible.
Alkali is neutralized by acid. Everyone should know this, so the second step after using the alkaline composition is to neutralize the alkaline composition.
Acidic
If we see salmon eyebrows — do not trust advertising that they can be removed in one procedure. Salmon can be removed in several procedures with acid removers. After color inversion (after laser removal) and taking into account the pigment depth and tattoo technique, we can assume how many procedures will be required to remove “salmon eyebrows”.
The toughest acid composition contains more than 10% glycolic acid. This is CORONA REMOVER. It must be diluted. The pH should be in the formulations preferably not lower than 3.5, if 3 — then this is already very much. For example, if you work with acid on people’s faces and do peeling, not lower than 1, but higher — this is already an aggressive acid. Tattoo master works with damaged skin.
What happens when a remover enters the skin?
At the molecular level, substances move, the reaction occurs with particles of pigment, with water, which is in our skin;
The chemical reaction may not be noticed (a lot depends on the composition of the pigment). Knowledge of which substance to use in the right situation and removal protocols are provided during the training course.
Like dissolves in like.
This principle should be remembered in order to understand the chemical removal process. In order to dissolve the pigment residues in the skin, it is necessary to break the intermolecular bonds between the pigment molecules and the drug molecules.
When do pigment particles dissolve in removers? Answer: when there is a mess in the system.
For dissolution (chemical reaction), in addition, it is necessary to break the hydrogen bonds between water molecules.
Quite privately, we can use the drug, but do not observe a positive result. There is no reaction to pigment.
The reason is the wrong choice of composition for removal, a chemical reaction between the pigment molecules and the composition does not occur and hydrogen bonds are not formed.
Removal (dissolution of the pigment by using a specific remover) in the skin — is determined by physical dissolution, which results in the dissolution of the pigment in remuevre and the skin and the breaking of intermolecular bonds (including hydrogen).
Only molecular substances (organic components of the dye in the skin) after a time and with a shallow introduction of pigment can physically leave (after tattooing). Non-mineral components are easily removed by laser.
A solvent is a substance that is larger in a chemical solution.
Other components of remuevre can be called solvent components.
The water in the skin can also be called a solvent, even if it is less than a solvent.
Physical dissolution occurs not only as a result of the formation of hydrogen bonds, but also as a result of the formation of other types of intermolecular bonds between particles of the remover and dye in the skin. If you remove the desired solvent from the remover, then we will not see the desired reaction of the pigment particles to the solution when using the composition. Therefore, in the process of tattoo removal, it is important to have knowledge of which remover to use in specific cases of removal.
IMPORTANT MATERIAL
The concentration of the remover is a solution that is in equilibrium with the soluble substance.
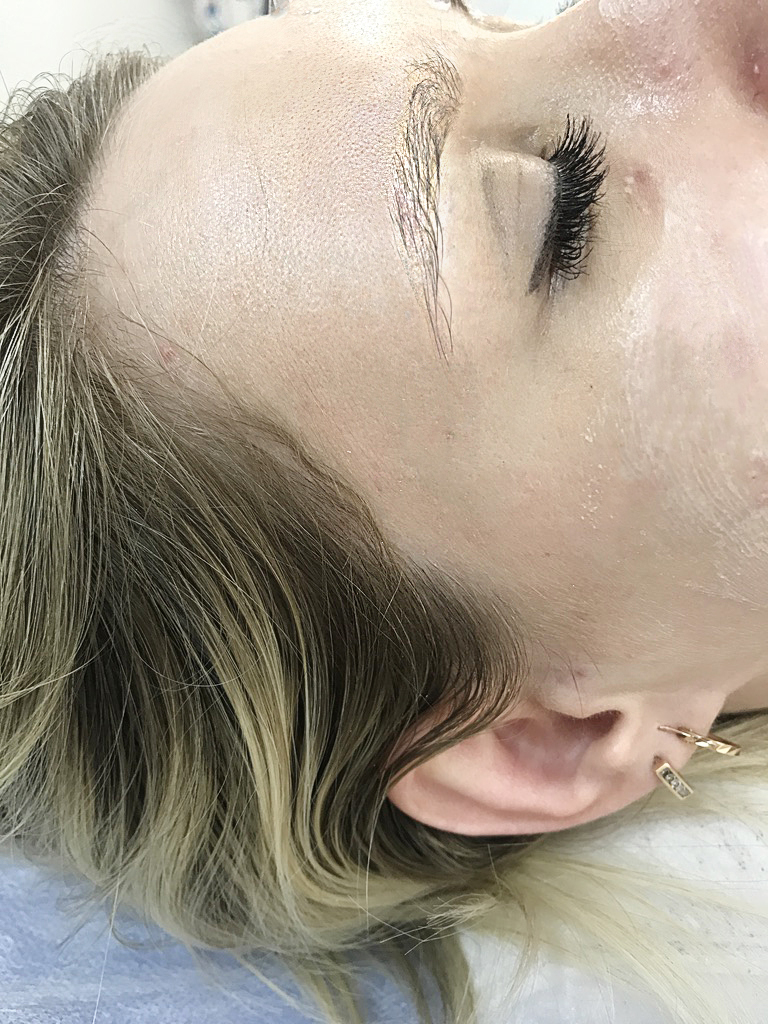
photo after removing yellow eyebrows. healed result. a mask on the client’s face
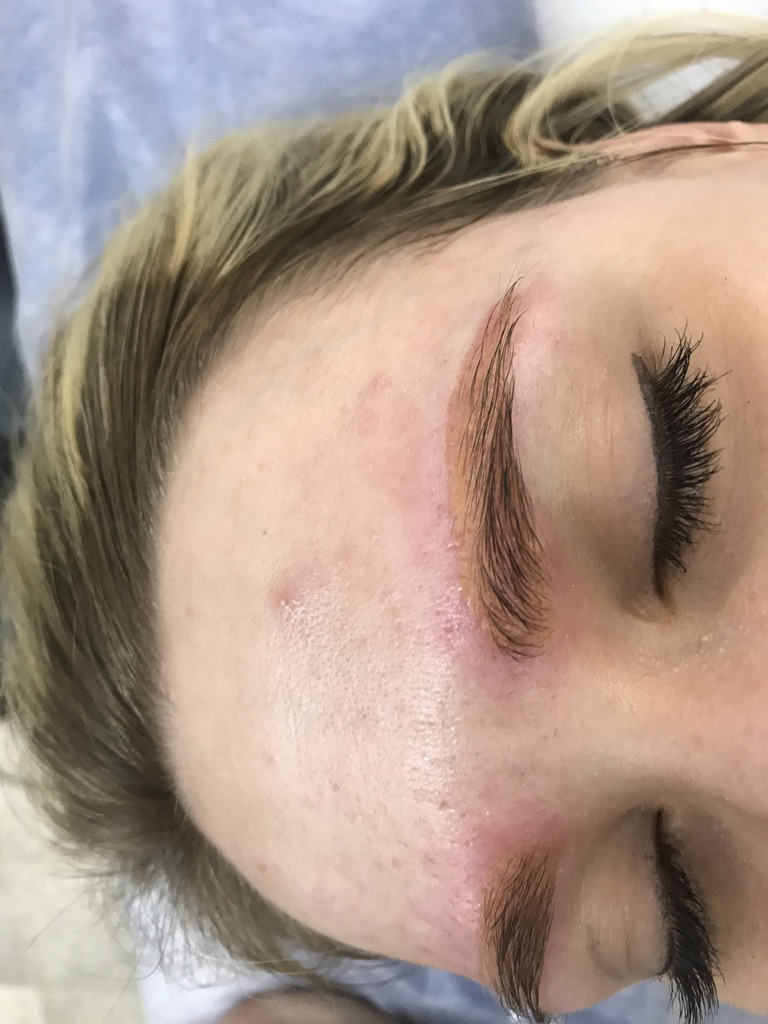
photo before removing yellow eyebrows. after using remover
The solubility of the substance can be more or less strong, characterizes the saturation composition of the remover. Reducing the concentration of the remover — we reduce the solubility. Solubility characterizes the composition. Therefore, removers can be divided into soluble, sparingly soluble (for example, remover removing, magic remover) and slightly soluble (alcohol removers, which are used in certain cases, more often to discolor pigment).
Accordingly, many removers are divided into saturated (reguy) and less saturated (Sivak) under the same conditions of the composition of the substance.
As we know, the main part of the pigment is located in the papillary layer of the dermis. When the pigment is in the matrix of the dermis, then it gradually overgrows with a membrane of connective tissue cells. Pigment particles can be at different depths. Despite this, the main thing for the master is to deliver the composition of the remover to the dermis. We study in more detail about the depth of introduction and techniques for working with drugs in the training “Full course of permanent makeup removal”. Teacher Hook Natalia.

Important information from the color training course:
We repeat that the pigments are divided into organic and inorganic (metal oxides — iron oxides, titanium dioxide).
An example of organic pigments: Carbon Black (carbon black), saturated black; derivatives of naphthol and benzidine (yellow, yellow-orange, red), synthesized carmine (dark red color), copper phthalocyanines — green and blue colors.
Plastic pigments — the molecules of these pigments are chains of polymers.
Next, we consider what reactions occur in the skin when using a remover.
To get started, let’s get acquainted: HOW DO THE PIGMENTS RESPOND TO THE COMPOSITION OF THE REMOVER?
ALKALINE REMOVERS
Some alkaline removers are called manufacturers and masters of oxide, since the main active ingredient of the remover is calcium oxide (CaO — quicklime).
The manufacturer must indicate the composition on a jar of removers.
We will analyze some firms.
Popular Rejuvi, Sivaka Recolor IX remover, A.Shakova remover (AS-Pigments remover, Alina Shakhova remover), Pigment Off remover (represented as non-acid). In all alkaline removers presented, the main active ingredient is calcium oxide (CaO).
Calcium oxide is chemically active, reacts vigorously with water, generating a large amount of heat:
CaO + H2O = Ca (OH) 2;
reacts with non-metal oxides: CaO + SO2 = CaSO3;
soluble in acids: CaO + 2HCl = CaCl2 + H2O.
It turns out:
in the interaction of simple substances: 2Ca + O2 = 2CaO;
during thermal decomposition of hydroxide and salts of certain oxygen-containing acids: 2Ca (NO3) 2 = 2CaO + 4NO2 + O2.
Calcium oxide does not interact with metal oxides, so we can not conclude that it destroys particles of pigment, as described by the manufacturer and was previously approved in many schools to remove permanent makeup. Calcium oxide, which is contained in the remover, reacts with water (it is contained in lymph. CaO + H2O = Ca (OH) 2. Lymph is a component of the body’s internal environment, a type of connective tissue, which is a transparent fluid; lymph that is secreted from small wounds are colloquially referred to as Sucrowitz)
As a result, the client sometimes feels a burning sensation in the area of removal, since according to the chemical formula a large amount of heat is released and during this reaction calcium hydroxide — Ca (OH) 2 is formed
Thus, after the reaction, a dry white powder is obtained, which we observe in the process of permanent makeup removal. The powder consists of small crystals, and when interacting with water, we observe the dissolution process.
Calcium oxide is involved in the breakdown of fats and the absorption of moisture.
Hydroxide (hydrated lime) — absorbs carbon dioxide and hardens, forms calcium carbonate. Therefore, in the process of removal with a remover (in this case, alkaline), we observe whitening on the skin and the instant formation of a white crust.
Therefore, permanent make-up artists should understand that the concentrated composition of hydrated lime (the composition of an alkaline remover) is not advisable to leave on the client’s skin during the removal process and afterwards for more effective dye removal, as this can cause burns on the skin.
ACID RESOURCES
have a number of common characteristics. Below is a table of indicator inversions when using different compositions.
Coloring some indicators in different environments
Indicators (inverse compositions) are also present in the chemical compositions of organic pigments. Indicators are substances that can reversibly change depending on the solution (alkaline or acidic).
As indicators, most often in practice they use:
methyl orange
litmus,
phenolphthalein,
universal indicator.
1) Metilorange with a neutral environment (in aqueous solutions) will have an orange color, litmus — purple.
In an acidic environment, both methyl orange and litmus acquire a red color (removal of an orange and violet organic composition with an acid remover).
Therefore, removing salmon eyebrows or violet with an acid remover as a result, we can get a red tint, alkaline — blue or yellow.
Recall the rules for mixing colors! What two colors can I get orange from when mixed? Of red and yellow. Indeed, in an alkaline environment, the methyl orange becomes yellow.
Red and blue colors when mixed give purple. Litmus in an alkaline environment turns blue.
2) If phenolphthalein was present in the pigment, then in an alkaline medium it acquires a bright neon raspberry color. When using other removers, it is colorless. Composition (phenolphthalein) is a colorless crystal, poorly soluble in water (practically insoluble in water, and therefore does not leave the skin), but is well soluble in alcohol. Conclusion: to remove the neon raspberry color, alcohol remover is good.
Therefore, it is recommended to remove raspberry eyebrows with an alcohol remover.
Acid Removers:
change the color of the organic dye;
react with inorganic pigments;
react with bases and amphoteric hydroxides;
react with basic and amphoteric oxides;
react with salts.
ACID REMOVER + METAL OXIDES
Acids interact with metals in the order of activity of metals to the left of hydrogen. As a result of the reaction, salt is formed and hydrogen is released.
1. Oxide (inorganic pigment) + acid remover
Removal of «salmon, green» eyebrows
What happens as a result of using an acid remover for pigment residues in which inorganic (mineral) components are present?
The interaction of acid and base with the formation of salt and water is called the neutralization reaction. Typically, such reactions proceed with the release of heat.Между оксидами металлов и кислотным составом могут протекать реакции обмена, в результате которых образуется соль и вода. Эту реакцию можно использовать для получения солей тех металлов, которые непосредственно с кислотой не реагируют.
It is important to understand that metal oxides and dioxide, which are in the composition of pigments, exhibit duality of properties. Depending on the conditions, they may have base properties or acid properties.
In an acidic environment (using an acid remover), inorganic pigments exhibit the properties of basic oxides. They react with acids like alkalis, forming salts and water. Reactions are easy, removers easily cope with “salmon” eyebrows (if the pigment remains inorganic).
White color (titanium dioxide), green (chromium oxide) and other inorganic residues are quite well removed by acid compounds.
2. Organic pigment + acid remover
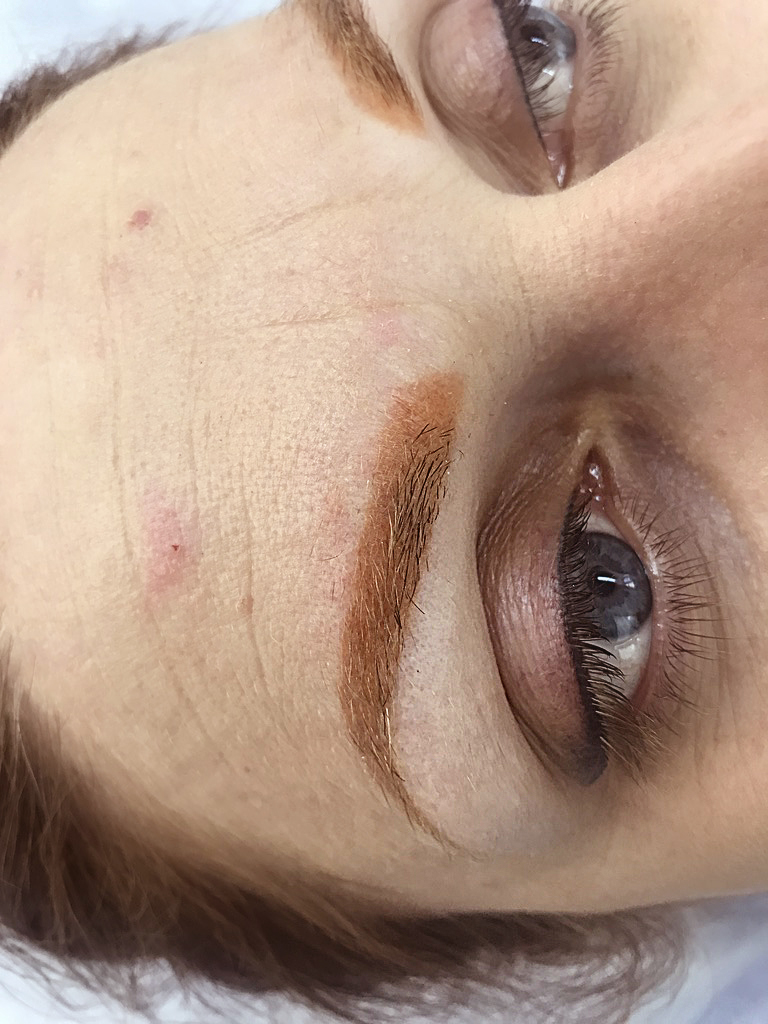
photo before removing salmon eyebrows
Carbon black. At the heart are cyclic closed chain hydrocarbons (cycloalkanes). Cycloalkanes include compounds with a diverse structure. Their molecules can contain one cycle (monocyclic alkanes) or several (polycyclic alkanes), can differ in the number of carbon atoms in the cycle and in the method of connecting the cycles.
Cycloalkanes are stable substances, they are difficult to enter into any reactions, can interact only with very strong acid removers, but this is dangerous for the skin. It is possible to remove with a laser, but again, we may encounter certain difficulties. Therefore, the bright black arrows must be removed by different systems that we train in practice.
But in addition to cycloalkanes, manufacturers can use another base for black pigments — manganese oxides, iron oxide (inorganic base), soot, graphite (organic base).
If the basis of the organic pigment is created from synthetic carmine (a red dye obtained from carminic acid), an analog of cycloalkanes (found in plants, causing red, violet and blue colors in nature — leaves, flowers) — then these substances are unstable to acids.
The red colors of organic dyes are more difficult to remove, repeated removal sessions are required.
Blue, green colors — difficult to remove — you need a system.
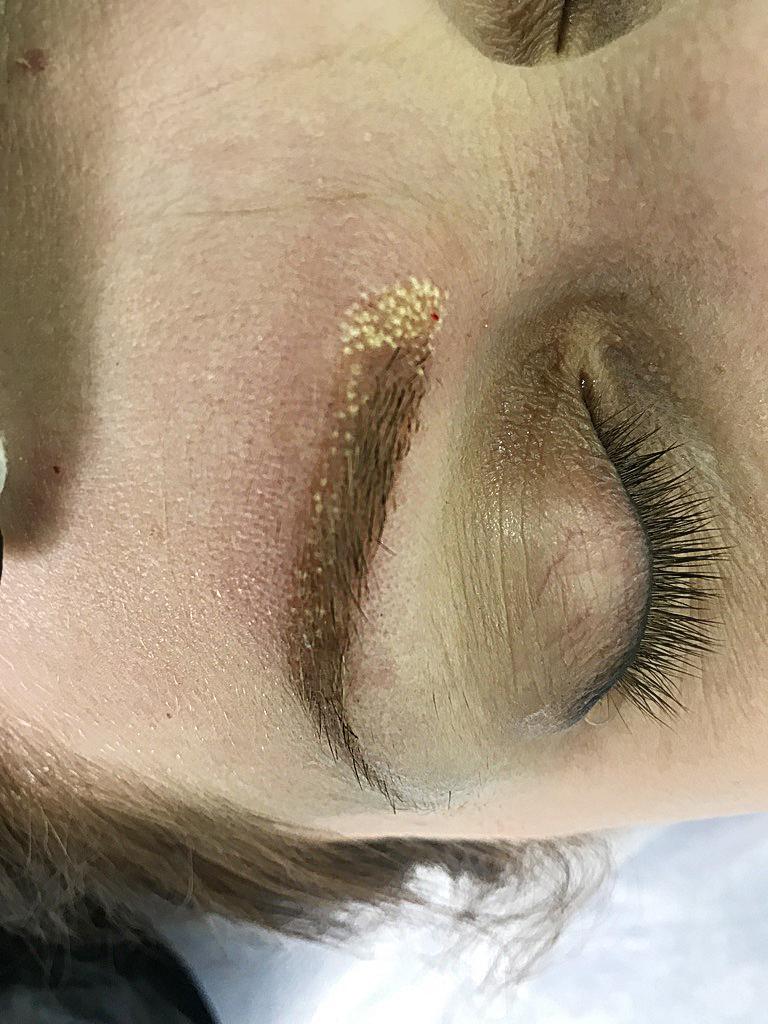
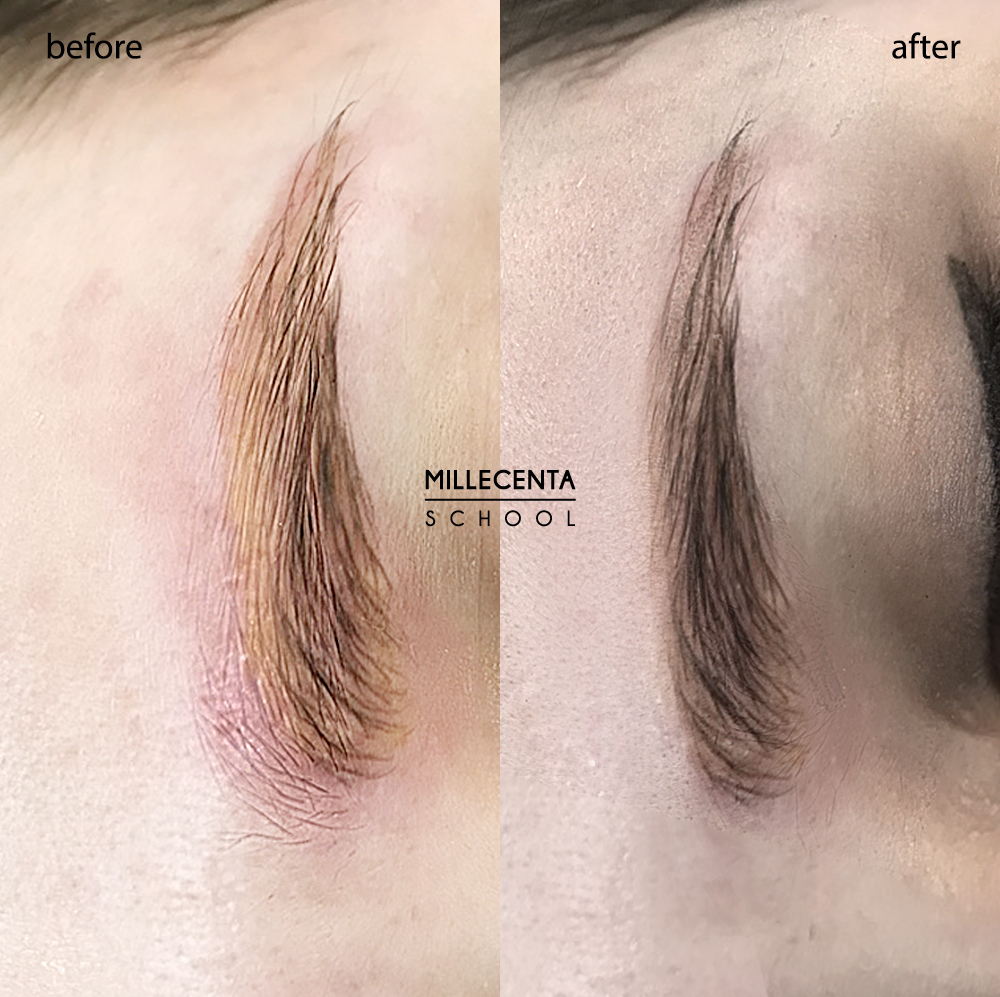
salmon eyebrow removal process (yellow-orange eyebrows)
3. Organic pigment + inorganic.
Carbon black
Removal the first time. Many customers are counting on this. Many craftsmen are afraid to work with oxide pigments, and customers are afraid that over time, the eyebrows will change color. But this is a normal reaction, since if viewed from the point of view of removing the correction, the oxide can be removed with a laser, in contrast to organic compounds.
If the master works with a milder acidic remover, then neutralization in many cases is not necessary. In other cases (if the pigment is deep, the styling is dense), it is advisable to neutralize.
TIP TO MASTERS: if the pigment residues on the eyebrows have any other residue than gray and black, it is better to start the removal process from the remover to prevent pigment inversion.
If the dark brown eyebrows after removal by a remover or a laser become gray or more saturated with gray and the laser exposure is increasingly becoming weak, the weak effect of the remover is the reason lies in the composition of black, the answer is that Carbon Black was used instead of black iron oxides. Carbon Black is very difficult to remove with both removers and a laser (it does not matter which company the laser will be). This polycyclic hydrocarbon is very resistant to acids, alkalis, and laser exposure.
Removal system: org. pigment + inorgan.

photo before — after removal of salmon eyebrows with remover
Red brown eyebrows
Let’s start with an acid remover, in which there is propylene glycol, an oxygen-containing chemical composition that will raise the molecular particles of the pigment, ruffle the protective barriers that surround the pigment.
Then we neutralize the effect of acid with a special preparation and calm the skin.
As long as the neutralizing substance remains on the skin at this time, a salt remover must be applied. Thanks to osmosis, it will pull particles of pigment to the surface of the skin. For this, you can use any salt remover.
Carbon Black is removed by acid + saline
Also, if we work with blue, green, turquoise residues — choose a remover. And we follow the acid + salt scheme. Copper phthalocyanine (significantly different in color and stability in organic solvents) is very stable (if you used a laser before), so you need to tune in to a long removal process.
Titanium dioxide
Consider the inversion of the pigment with titanium dioxide after laser removal.
There is a large amount of titanium dioxide in the labial pigments. And often brown eyebrows after laser removal can turn dark green (with a blackish tint) or black. After using laser equipment, the structure of the composition (which contained titanium dioxide, namely, crystals — and their light reflecting ability) changes.
Iron oxides together with titanium dioxide are very often found in eyebrow pigments. When using an acid remover with an alternating laser — as a rule there are no questions about removal. Pigment inversion can occur only after the first laser removal. Therefore, if lips are removed, then it is best to use an acid + salt system.
BLACK SPOTS AFTER REMOVING THE LASER, BLACK SPOTS ON EYEBROWS AFTER 1 procedure — what is it?
A very important problem that worries many masters is the change in the composition of polymers that have changed their composition under the influence of a laser. This reaction can be called fusion. We often observe blots after removal or a black-green pigment and do not understand why this happened. Removing such molten spots is very difficult.
Polymers are very often used in the manufacture of dyes.
Polymers
— These are high molecular weight chemical compounds whose macromolecules are composed of many units. The polymer molecules are characterized by a huge molecular weight. There are several classification options.
The best option for removing modern pigments is to combine acid and osmosis (saline).WHAT SHOULD BE PRESENT IN THE STRUCTURE OF THE REMOVER?
Organic acids (+ additional substances);
Glycolic acid (reacts with a pigment, helps smooth out scars, and if you add other components, it can be used to treat deeper scars);
Lactic acid (enhances the action of glycolic acid, as well as high activity in the fight against dye particles);
Additional components reduce the friction of the needle and reduce the invasiveness of the session;
Substances that are added to regenerate the skin, or additional care products after removal.
COMPLETELY REMOVED SANDWICHES
Consider an example. If the tattoo is blocked several times and very deep with different pigments, removal will be difficult. It is better to start with a remover and clean the skin with a laser, then the removal process will be faster. The client should tune in that for removal it will be necessary to carry out up to 10 procedures with a frequency of 1 -1, 5 months.
The opinion of many masters is that acid removers work on whitening, and that the client will immediately get the result. This opinion is erroneous. The results after removal can be completely different. Sometimes the pigment becomes brighter, sometimes a color change occurs and different removal systems are needed. Most oxide pigments are removed in 1 — 2 procedures, but the residues of the organic dye require a multiple approach. Always to remove irresistibly several procedures.
LIKE FORM, does not like the color — too dark, the solution is to lighten the first session, the second — overlap
Expectation and reality always set a barrier in time. If the client insists — enter the drug deeper, more, we are familiar with such situations — do not strain and go on about the client. Be sure to fill out the written consent of the client for clarification and removal. In work with removers faster and more does not work.
The client must understand this and the master too. Therefore, for those who live abroad, it is easier to train the master and send a client to him for removal, since removal is not possible in 1 procedure.
CONCLUSIONS
So to summarize.
Salt removers are electrolytes. In solutions, salt removers dissociate into positively charged metal ions (cations) and negatively charged ions (anions) of acid residues.
They can interact with acidic ones; as a result, an exchange reaction occurs during which a chemically more active acid displaces a less active one.
Some salt removers can interact with alkaline ones, but an exchange reaction will be possible if at least one product is practically insoluble (precipitates).
In alkaline removers, the main active ingredient is calcium oxide (CaO).
Calcium oxide is chemically active, reacts vigorously with water, generating a large amount of heat:
CaO + H2O = Ca (OH) 2;
reacts with non-metal oxides: CaO + SO2 = CaSO3;
soluble in acids: CaO + 2HCl = CaCl2 + H2O.
It turns out:
in the interaction of simple substances: 2Ca + O2 = 2CaO;
during thermal decomposition of hydroxide and salts of certain oxygen-containing acids: 2Ca (NO3) 2 = 2CaO + 4NO2 + O2.
Calcium oxide does not interact with metal oxides, so we can not conclude that it destroys particles of pigment, as described by the manufacturer and was previously approved in many schools to remove permanent makeup. Calcium oxide, which is contained in the remover, reacts with water (it is contained in lymph. CaO + H2O = Ca (OH) 2. Lymph is released — lymph — a component of the body’s internal environment, a type of connective tissue, is a clear liquid; lymph, which stands out from small wounds in colloquial name)
As a result, the client sometimes feels a burning sensation in the area of removal, since according to the chemical formula a large amount of heat is released and during this reaction calcium hydroxide — Ca (OH) 2 is formed
Thus, after the reaction, a dry white powder is obtained, which we observe in the process of permanent makeup removal. The powder consists of small crystals, and when interacting with water, we observe the dissolution process.
Calcium oxide is involved in the breakdown of fats and the absorption of moisture.
Hydroxide (hydrated lime) — absorbs carbon dioxide and hardens, forms calcium carbonate. Therefore, in the process of removal with a remover (in this case, alkaline), we observe whitening on the skin and the instant formation of a white crust.
Therefore, permanent make-up artists should understand that the concentrated composition of hydrated lime (the composition of an alkaline remover) is not advisable to leave on the client’s skin during the removal process and afterwards for more effective dye removal, as this can cause burns on the skin.
Calcium hydroxide Ca (OH) 2 (hydrated lime, fluff) — a white crystalline substance, crystallizes in a hexagonal crystal lattice. When heated to 580 ° C, it decomposes:
Ca (OH) 2 = CaO + H2O.
It is slightly soluble in water and is a strong base.
Reacts with acids:
Ca (OH) 2 + 2HCl = CaCl2 + 2H2O;
with non-metal oxides:
Ca (OH) 2 + 2CO2 = Ca (HCO3) 2; Ca (OH) 2 + CO2 = CaCO3 + H2O;
participates in metabolic reactions:
3Ca (OH) 2 + 2FeCl3 = 2Fe (OH) 3 + 3CaCl2; Ca (OH) 2 + 2NH4Cl = CaCl2 + 2NH3 + 2H2O.
It is obtained by dissolving calcium and calcium oxide in water, with the interaction of calcium salts with alkalis:
Ca (NO3) 2 + 2NaOH = Ca (OH) 2 + 2NaNO3.
Calcium hydroxide and calcium oxide are substances that allow us to mix immiscible ingredients during the removal process, they are also called emulsions. Thanks to them, substances that were in the composition of the pigment move freely.
Benzoic acid is present in the composition of the removers (Shakhova’s remover, Sivak’s remover).
In these removers, no-acid acid fulfills its purpose of alkali neutralization.
Benzoic acid is used in medicine. Other components of removers perform auxiliary functions. For example, zinc oxide relieves inflammation, glycerin moisturizes and retains moisture, magnesium oxide (as part of removers) dries the skin.
Triethanolamine is a neutralizing agent, due to its presence in the remuver, the physicochemical properties and shelf life of the substance are improved. On the other hand, this substance is often criticized because of its potential toxicity and carcinogenicity. Let’s see: how appropriate is triethanolamine in the composition of the remover, how are its properties manifested, and how dangerous can it be for the skin?
Triethanolamine is a low molecular weight amino alcohol, which is widely used in the cosmetics industry as a base in the manufacture of soaps, an emulsifier of oils, as well as a buffering and neutralizing agent.
As part of the remover, it performs the functions of acting as a regulator of acidity, a cleaning and foaming agent. In the process of removal, it has a cleansing effect: with its help, contaminants dissolve. Promotes, so to say, leaching of pigment particles from the skin.
Triethanolamine is produced synthetically — it is obtained by the oxidative reaction of ethylene with ammonia. In its pure form, it is a viscous, colorless or pale yellow liquid, sometimes giving off the smell of ammonia. TEA is highly soluble in water, oil, alcohol, acetone, but slightly soluble in hydrocarbons and ethers. In combination with acids, forms salts.
Sodium hydroxide in the composition of the remover is able to corrode materials, organic matter and iron oxides; forms sodium salts of fatty acids, which have a detergent effect.
Potassium hydroxide (in a certain concentration) in the composition of the remover performs a cleansing effect, an acidity regulator, but in large quantities it can cause burns.
The action of the removers is based on the physical destruction of the particles of the coloring matter, when some of the particles leave with the lymph flow, part can be carried out into the scab, part is destroyed by phagocytes (cells of the immune system that protect the body by absorbing harmful foreign particles, as well as dead or dead cells). In this regard, skin restoration requires a long time — 2 months.
Removers (including CaO) are effective, but require delicate work by the master in the process of removing permanent makeup. The master and the client will need several procedures to remove the pigment, since if removed in one procedure, you can make burns on the skin of the client. Alkaline removers are single-phase, and acidic 1-2-3-phase.
ACID
change the color of the organic dye;
react with inorganic pigments;
react with bases and amphoteric hydroxides;
react with basic and amphoteric oxides;
react with salts.
may consist of several phases.
Many masters give an acid preference in work, because of the flexibility to use to remove permanent makeup.
Acid removers are less traumatic than alkaline ones, and when used properly, they can cope well with residues of organic companions that are not removed by laser equipment.
